July/August 2014
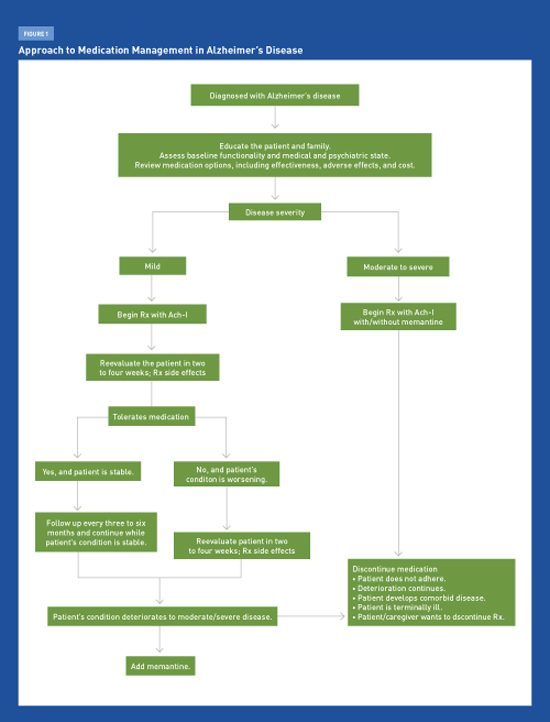
Therapeutic Options in Alzheimer’s Disease Physicians must discuss with patients and caregivers the available options using medications to treat Alzheimer’s disease, including their limited clinical benefits, adverse side effects, and associated costs. Alzheimer’s disease (AD), the most common form of dementia, affects approximately 5.4 million Americans and is projected to impact three times the current number by 2050. Amyloid plaques, neurofibrillary tau tangles, and the depletion of acetylcholine in the brain are among the pathologic manifestations of AD, which causes progressive memory loss and cognitive decline. The diagnostic approach to the AD continuum was described in the January 2014 Journal of the Arkansas Medical Society article “Diagnosing Alzheimer’s Disease: Update for the Primary Care Clinician.” The primary focus of this article is to describe the currently available therapies. The FDA-approved AD medications currently available for marketing are the acetylcholinesterase inhibitors (Ach-Is) and memantine (Namenda). Although these medications can slow the progression of AD symptoms, no pharmacologic agents can prevent, delay, or reverse the progression of the disease itself. Vitamins, food supplements, and gingko biloba extract have not been found to be effective. Nonpharmacological therapies are affordable complementary approaches to medications that can aid in managing patients with AD. Primary care physicians will continue to play an increasingly greater role in the early initial diagnosis and providing support and counseling for AD patients, as well as in formulating management strategies as the disease progresses. After initial diagnosis, the patient’s family should be referred early to local support groups, and the eventual medico-legal issues such as driving and end-of-life planning should be appropriately addressed. Physicians should discuss with patients and caregivers the options related to treating AD with medications, including their modest clinical benefit, adverse effects, and associated costs. Table 1 provides a summary of recommended medications used in the treatment of AD. Medications Ach-Is Ach-Is are first-line agents for treating mild to moderate AD.1,3,4 Donepezil and rivastigmine transdermal are the only Ach-Is specifically labeled for patients with severe AD (ie, with Mini-Mental State Examination scores of less than 10). Donepezil is the least expensive Ach-I and is available in generic form. Current evidence indicates that Ach-Is may improve cognitive function, behavior, and activities of daily living in patients with AD. Despite showing statistical significance in various studies, the actual clinical effects of Ach-Is are marginal at best, with no observed large treatment effect. No systematic evidence exists for efficacy differences among the three Ach-Is. There is limited evidence on the long-term beneficial effects of Ach-Is, as most efficacy studies have been conducted in three- or six-month trials.1 Thus, the use of Ach-Is involves balancing the modest expectations of benefit with the potential for the drugs’ adverse effects and the need for considerable clinical judgment. It is reasonable to discontinue Ach-I treatment if symptom progression continues at the same rate as that prior to initiating treatment. Therapy may be restarted if symptoms worsen after tapering medications. The most common adverse events due to Ach-Is are cholinergically mediated and include nausea, diarrhea, vomiting, anorexia, bradycardia, and weight loss.1-3 Muscle cramps are common with donepezil. In general, more frequent adverse events are observed with higher dosage formulations compared with lower dosage formulations. Rashes have been reported with transdermal rivastigmine formulations, and some patients experience side effects similar to those associated with the oral form. The efficacy of transdermal patches likely is lower unless administered in higher doses. Few trials have directly compared Ach-Is with respect to adverse events, and the long-term safety of their use has not been systematically studied. However, patients on Ach-Is should be monitored for the occurrence of bradycardia and syncope, as one large study cohort has shown a “modestly greater risk of bradycardia in patients with dementia taking Ach-Is than those not taking these drugs. In patients taking donepezil, the risk of bradycardia may increase with increasing doses.”5 “Among older patients, initiation of cholinesterase inhibitor therapy was associated with more than a doubling of the risk of hospitalization for bradycardia. Resumption of therapy following discharge was common, suggesting that the cardiovascular toxicity of cholinesterase inhibitors is underappreciated by clinicians.”6 Early cholinergic effects frequently are related to the medications’ initial dosing and titration. Reducing the dose temporarily and retitrating up gradually may reduce the reemergence of cholinergic adverse events. Many patients tend to become tolerant to the adverse events. Other potential side effects include an increased risk for gastrointestinal bleeding, especially in patients concurrently using anti-inflammatories. Memantine Two of three controlled studies in moderate to severe AD showed small but statistically significant effects on cognition and function and a very small effect on behavior.7,8 (Patients on memantine were slightly less likely to develop agitation, though there was no evidence of an effect on agitation already present.) Studies of memantine in mild to moderate AD have shown no significant benefits. Memantine is used in 10-mg twice-per-day dosing, and the renal dose is 5 mg twice per day. A once-daily, 28-mg, extended-release formulation of memantine was approved by the FDA in June 2010 but only recently has been marketed in the United States. Adverse events may be infrequent but can include headache, dizziness, confusion, somnolence, and infrequent hallucinations. In clinical practice, memantine is prescribed either alone or in combination with an Ach-I, often after the latter has been used for a period of time.9 Memantine’s duration of effectiveness is unknown beyond the six-month length of the clinical trials, although it tends to be prescribed for indefinite periods. Antipsychotics Most deaths are related to cardiovascular or infectious causes, possibly because antipsychotics can prolong QT interval and cause sedation, which may increase the risk of aspiration. Other common adverse effects include gait disturbances and extrapyramidal effects. Using atypical antipsychotics to treat behavioral and psychological symptoms of dementia (BPSD) in patients with AD generally should be avoided because of adverse effects, although these agents may be appropriate in some rare situations. Anticonvulsants Efficacy Controversies Despite trends showing marked statistical significance, it is clear that there is substantial overlap in outcomes between drug and placebo patients in trials. Thus, it is difficult to identify the patients who benefit from Ach-Is or memantine because the outcome measures and mean changes on scale scores do not clearly identify responders. Placebo-controlled clinical trials generally have lasted for six months, with a few lasting up to 12 months or longer.1-3 Inferences are made on whether these medications will continue to be beneficial far longer and perhaps indefinitely. Over the long term, however, as patients inevitably worsen, it becomes even more difficult to determine whether any given individual is benefiting from the drugs. Thus, duration of treatment remains an unresolved issue. Discontinuing Ach-Is has been associated with worsening of cognition and confusion in some patients.4 Thus, in these patients, the decision focuses on whether to continue medication or to taper gradually and discontinue when physicians are uncertain of the ongoing benefit. It is generally good practice to taper these medications before discontinuing, even though both donepezil and memantine have long terminal half-lives. Despite clinical practice, studies using Ach-Is and memantine specifically to treat agitation and disruptive behavior are limited. Further, clinicians must be sure that the Ach-Is are not exacerbating restlessness, agitation, or sleeplessness. Nonpharmacologic Therapies Multicomponent interventions based on caregiver education and support have delayed the institutionalization of patients with AD, with only modest amounts of resources used. This important outcome with respect to both quality of life and cost was not found with any other treatment approach on the basis of high-quality evidence. For other outcomes (cognition, activities of daily living, behavior, and mood), the magnitude of the effect appeared to be similar to the effect obtained by drugs. Because of the general absence of side effects and their ability to be more readily individualized, NPTs are preferable when particular activities of daily living or behaviors are targeted. However, rather than being viewed as an alternative to medications, NPTs should be understood as complementary approaches to medications. NPTs lacking any recommendations include transcutaneous electrical stimulation, physical exercise, music, reminiscence, massage and touch, recreation therapy, light, multisensory stimulation, support and psychotherapy, validation, case management, and respite care. A recent consensus statement from the National Institutes of Health’s State-of-the-Science Conference on Preventing Alzheimer’s Disease and Cognitive Decline concluded that current evidence is insufficient to support the association of any modifiable factor, pharmacologic agent, or dietary supplement with a reduction in the risk of AD.13 Conclusion — Priya Mendiratta, MD, MPH, is codirector of the geriatric clerkship program and an assistant professor at the University of Arkansas for Medical Sciences (UAMS) Donald W. Reynolds Institute on Aging in Little Rock. — J. Y. Wei, MD, PhD, is a professor and the chair of the department of geriatrics in the UAMS College of Medicine. She also is executive director of the Reynolds Institute on Aging. — Mark Pippenger, MD, is an associate professor in the departments of geriatrics and neurology at UAMS. A neurologist with additional training in dementia and memory loss, he also is codirector of the memory center at the Reynolds Institute on Aging.
Table 1: Medications for Alzheimer’s disease
1Long-acting (elimination half-life 70 hours; steady state in two weeks after starting), metabolized in liver and excreted unchanged in kidneys. Clinicians should be cautious, however, not to use 20 mg of generic donepezil in place of the 23-mg branded dose, as the formulations are different. The higher 23-mg dose rarely is tolerated due to significant side effects and also is costly with marginal benefit.The half-life of the compound is approximately seven hours. It is metabolized by the liver and excreted in the urine. — Sources: References 2, 3, 8, and author Mendiratta; generic and brand prices based on information from Walmart.com Table 2: Other Therapeutic Candidates Used for Treating Alzheimer’s Disease
Information in table adapted from reference 3 References 2. Schneider LS, Sano M. Current Alzheimer’s disease clinical trials: methods and placebo outcomes. Alzheimers Dement. 2009;5(5):388-397. 3. Schneider LS. Alzheimer disease pharmacologic treatment and treatment research. Continuum (Minneap Minn). 2013;19(2 Dementia):339-357. 4. Winslow BT, Onysko MK, Stob CM, Hazlewood KA. Treatment of Alzheimer disease. Am Fam Physician. 2011;83(12):1403-1412. 5. Hernandez RK, Farwell W, Cantor MD, Lawler EV. Cholinesterase inhibitors and incidence of bradycardia in patients with dementia in the Veterans Affairs New England healthcare system. J Am Geriatr Soc. 2009;57(11):1997-2003. 6. Park-Wyllie LY, Mamdani MM, Li P, Gill SS, Laupacis A, Juurlink DN. Cholinesterase inhibitors and hospitalization for bradycardia: a population-based study. PLoS Med. 2009;6(9):e1000157. doi: 10.1371/journal.pmed.1000157. 7. Reisberg B, Doody R, Stoffler A, et al. Memantine in moderate-to-severe Alzheimer’s diease. N Engl J Med. 2003;348(14):1333-1341. 8. Tariot PN, Farlow MR, Grossberg GT, et al. Memantine treatment in patients with moderate to severe Alzheimer disease already receiving donepezil: a randomized controlled trial. JAMA. 2004;291(3):317-324. 9. Porsteinsson AP, Grossberg GT, Mintzer J, Olin JT; Memantine MEM-MD-12 Study Group. Memantine treatment in patients with mild to moderate Alzheimer’s disease already receiving a cholinesterase inhibitor: a randomized, double-blind, placebo-controlled trial. Curr Alzheimer Res. 2008;5(1):83-89. 10. Ballard C, Waite J. The effectiveness of atypical antipsychotics for the treatment of aggression and psychosis in Alzheimer’s disease. Cochrane Database Syst Rev. 2006;(1):CD003476. 11. Gauthier S, Cummings J, Ballard C, et al. Management of behavioral problems in Alzheimer’s disease. Int Psychogeriatr. 2010;22(3):346-72. 12. Ballard C, Khan Z, Clack H, Corbett A. Nonpharmacological treatment of Alzheimer disease. Can J Psychiatry. 2011;56(10):589-595. 13. Daviglus ML, Bell CC, Berrettini W, et al. NIH state-of-the-science conference statement: preventing Alzheimer’s disease and cognitive decline. NIH Consens State Sci Statements. 2010;27(4):1-30. |